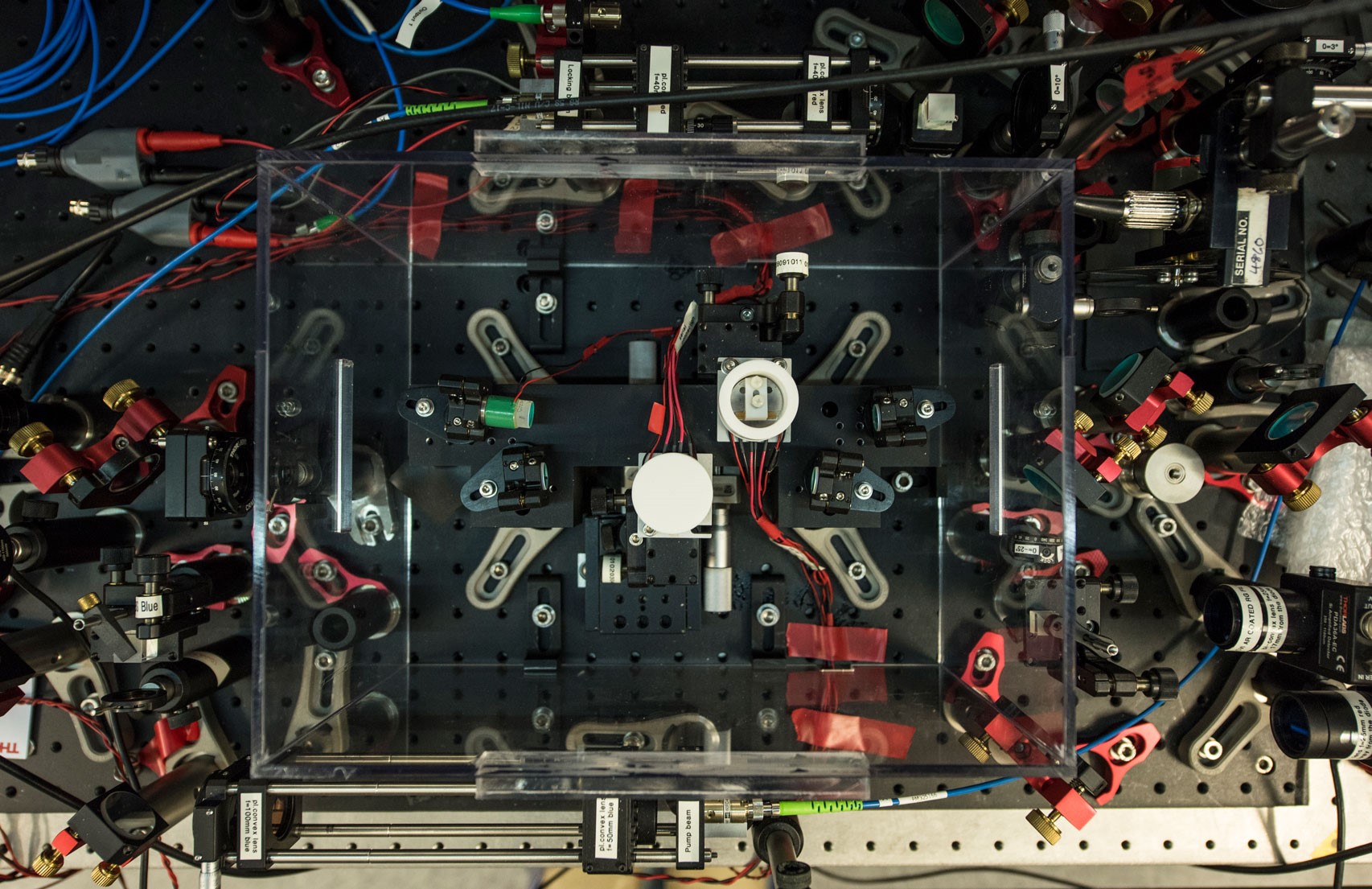
This chamber holds cold atoms, which can work as the microscopic elements of quantum technologies.
We have to be sure that in the chamber there are only the atoms we are interested in, without impurities: that's why we extracted all the air that was inside, creating vacuum inside.
This is one of the coldest corners of the planet: only a small fraction of the absolute zero (micro- or nano- Kelvin).
There is no fridge that can get as cold as that: we need powerful lasers and magnetic fields to cool down and trap the atoms.
There is no thermometer able to measure such low temperatures: at these levels, we have to estimate the temperature by observing with a camera how fast the atom cloud expands.
You can't touch the atoms, but you can use light to change or measure their state.
The atoms are the core of the experiment, but we need all the other elements of the lab to control them.
Here we have Rubidium atoms, but also many other elements of the first two columns of the periodic table (Potassium, Strontium, etc.) are used in cold atoms experiments. Scientists choose these because they have simpler electronic structures, i.e. it's as if they have only one or two electrons.
Atoms do not interact with any kind of light: given a certain element, only very specific light wavelengths can interact with it.